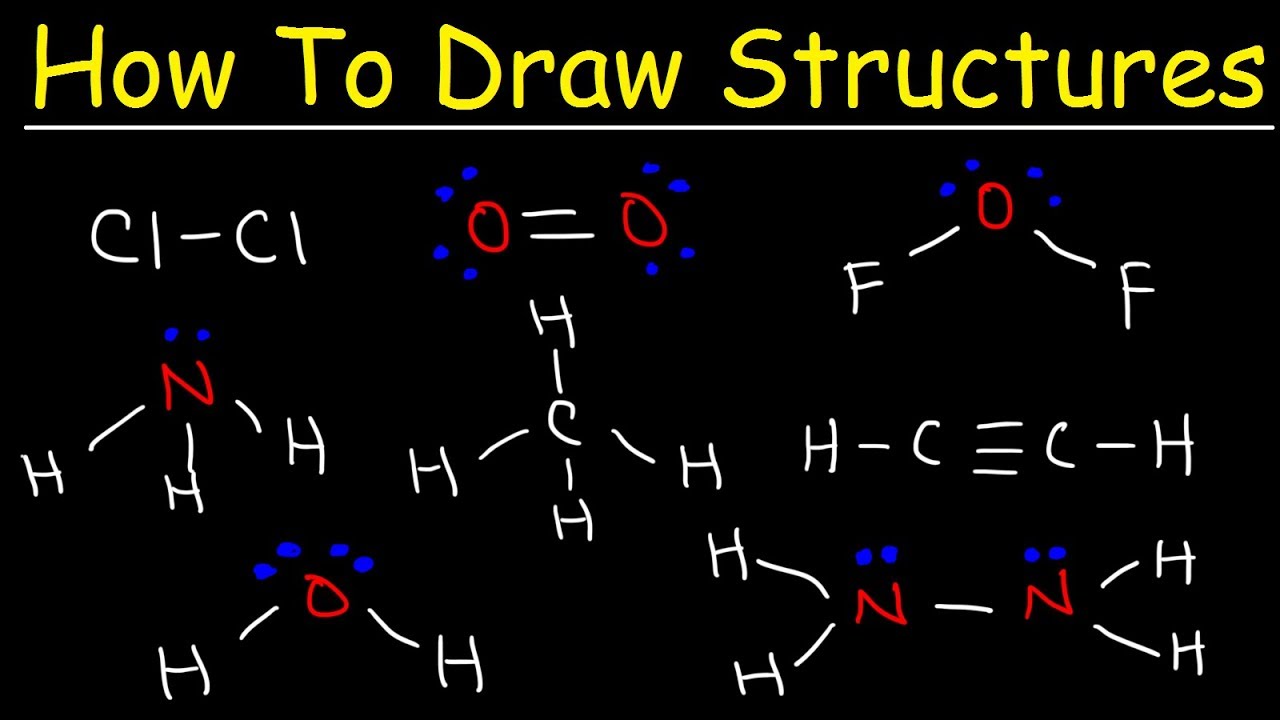
Dive into the fascinating world of Lewis structures, where the arrangement of electrons around atoms reveals the secrets of molecular bonding and geometry. This quiz will test your ability to represent molecules accurately and understand their underlying structures. Sharpen your pencils and get ready to draw your way to chemical mastery!
We recommend that you do not leave the page that you are taking this quiz in. Stay honest 🙂
Lewis Structure Quiz Questions Overview
2. Which of the following elements can have an expanded octet?
Carbon
Nitrogen
Sulfur
Oxygen
3. How many lone pairs of electrons are on the central atom in the Lewis structure of ammonia (NH3)?
0
1
2
3
4. What is the formal charge on the central atom in the Lewis structure of CO2?
0
1
-1
2
5. Which molecule has a trigonal planar shape according to its Lewis structure?
CH4
NH3
BF3
H2O
6. What is the bond order of the nitrogen-nitrogen bond in N2?
1
2
3
4
7. Which of the following molecules has a resonance structure?
H2O
CO2
O3
CH4
8. In the Lewis structure of sulfur hexafluoride (SF6), how many bonding pairs and lone pairs are around the sulfur atom?
6 bonding pairs, 0 lone pairs
4 bonding pairs, 2 lone pairs
5 bonding pairs, 1 lone pair
6 bonding pairs, 1 lone pair
9. Which of the following molecules has a bent shape according to its Lewis structure?
CO2
CH4
H2O
BF3
10. What is the electron geometry of the central atom in the Lewis structure of methane (CH4)?
Linear
Trigonal planar
Tetrahedral
Bent
We recommend that you do not leave the page that you are taking this quiz in. Stay honest 🙂
Can Your Friends Do Better Than You in This Quiz?
Share this quiz with your friends and compare results.
Was this page helpful?